AKTUELT
Nyheter &
Release notes
Our passion for innovation and improvement keeps us in constant motion, and we want to share the latest updates and news with you.
Vitalthings launches new contactless health monitor for primary healthcare
Vitalthings Guardian H10 is a new contactless health monitor developed to reduce the need for physical supervision and help solve some of the most critical capacity problems in municipalities.
Vitalthings AS acquire bankruptcy estate of Novelda AS
Vitalthings AS has acquired the bankruptcy estate of Novelda AS, a technology company known for its advanced ultra-wideband (UWB) radar sensors.
New clinical study: "Exceptional accuracy"
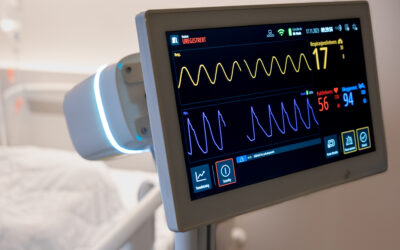
Vitalthings Guardian M10 has been validated in a new study at St. Olavs Hospital, demonstrating remarkable accuracy in respiratory rate measurements using contactless technology.
Press Release: Medical Approval for Guardian M10
Vitalthings has received European medical approval for its contactless patient monitor, Vitalthings Guardian M10, designed for continuous monitoring of vital parameters.
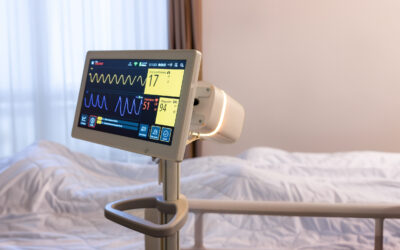
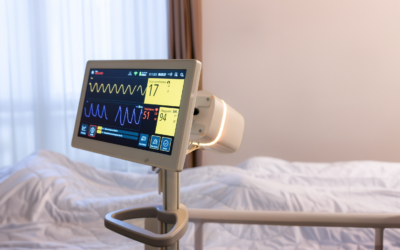
The world's first contactless patient monitor
Vitalthings Guardian M10 monitors respiratory rate contactless. The innovation partnership with St. Olavs Hospital ensures that we meet the needs of the healthcare system.
Heidi Blengsli Aabel becomes Commercial Director at Vitalthings.
Heidi Blengsli Aabel has been appointed as the Commercial Director at Vitalthings. Aabel has led and developed the health technology company CheckWare from 2008 to the present.
Press Release: Successful Emission
The health technology company VitalThings AS in Trondheim recently completed a capital raise of 41.6 million. The interest in the capital raise was significant, surpassing the target of 35 million.
Clinical trial at St. Olavs Hospital
A central milestone in the Autoskår project: We have now finished the clinical trials of the upcoming patient monitor at St. Olavs Hospital in Trondheim. This marks...
Vitalthings is ISO 13485:2016 certified
In a world where medical technology plays an ever more crucial role in healthcare, becomes the importance of ensuring quality and safety becomes ever more critical.
Project Autoskår
Through close collaboration between the business sector and healthcare, new technology is developed to enhance patient safety. Autoskår represents an innovation for the future.