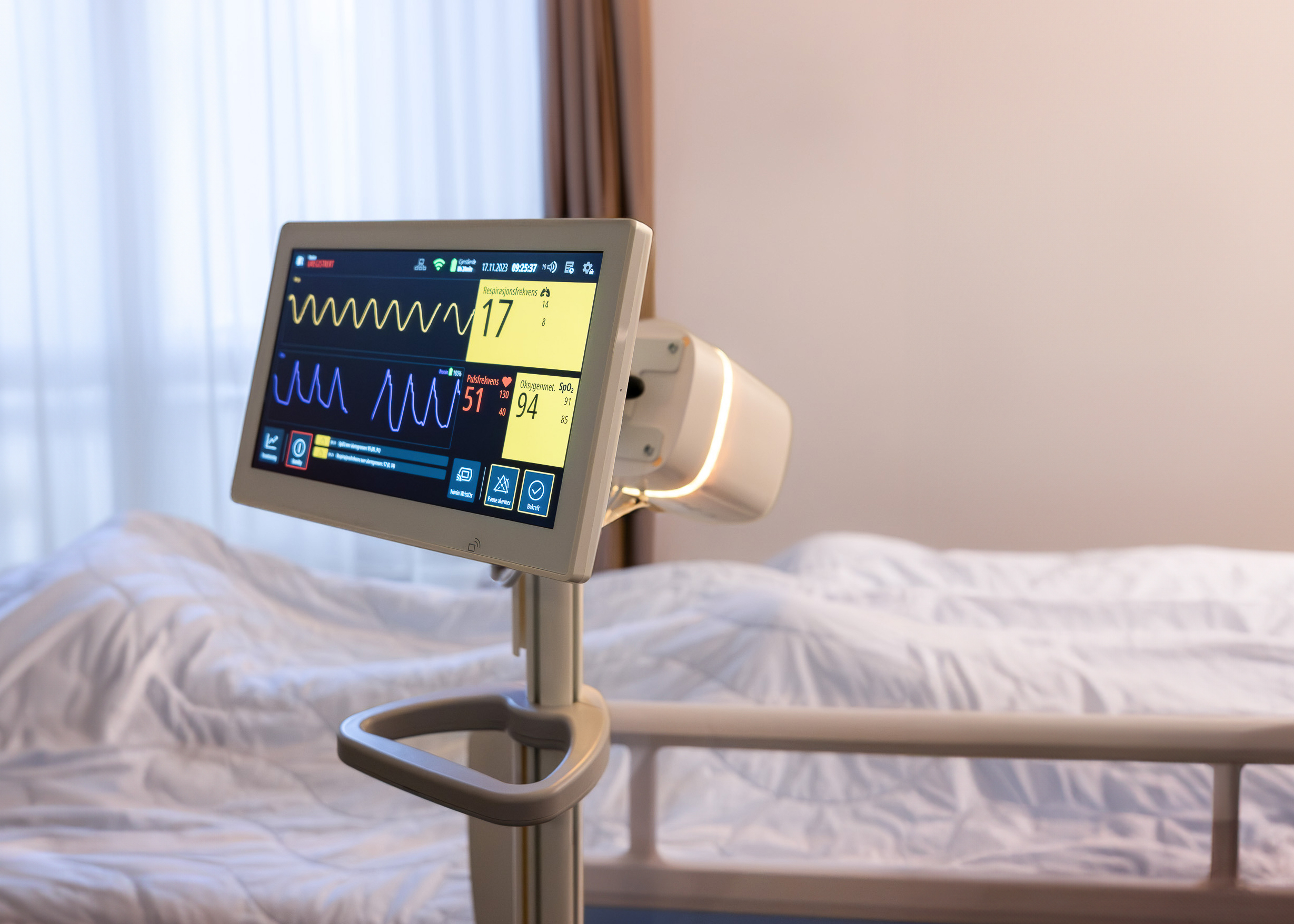
Vitalthings has received medical approval for contactless patient monitor.
The health technology company Vitalthings is launching its first medical product, the Guardian M10.
The solution measures respiratory rate and breathing patterns without the need for sensors on the patient's body and includes comprehensive alarm functionality for notifications of deviations from standards and personal profiles. The platform also handles pulse and oxygen saturation through wirelessly connected pulse oximeters and offers various integration options with both mobile alert platforms and patient records. The Guardian M10 has received European medical approval in class IIb, which means the system has documented safety and performance to be used, among other things, for monitoring vital parameters.
Since 2017, Vitalthings has had the digital night watch Somnofy on the market, and 40 municipalities and organizations are now using it. The company began developing its first medical product in 2020, with the main focus on the early detection of deteriorating health conditions. Respiratory rate and breathing patterns are crucial parameters in this. The solution, Guardian M10, was developed in close collaboration with St. Olavs Hospital in Trondheim.
Receiving European medical approval for what is likely the world’s first contactless patient monitor for continuous monitoring is a tremendous achievement and a significant milestone for Vitalthings. In just over three years, we have developed a product that enables automatic, continuous patient monitoring in environments where, today, such follow-up requires extensive manual labor—such as in emergency rooms, hospital wards, and nursing homes. The solution is also being further developed for use in home settings. For our customers, this represents a substantial potential for increased patient safety, reduced costs, and improved working conditions for their staff. There is significant interest in our solutions and our work, both domestically and internationally. I am proud and deeply impressed by our employees who have made this possible.
A number of studies have documented the positive effects of continuous monitoring on both patient safety and economic outcomes. However, the limitation has often been the available technology, which has either been unsuitable for long-term monitoring, relied on disposable equipment, or suffered from inaccuracies in measurements that lead to alarm fatigue. This is why it is exciting to see the possibilities that contactless patient monitoring will now provide, especially for vulnerable and demanding patient groups. A key focus during development has been the accuracy of clinical measurements. I look forward to the publication of the results from the clinical trials. These are results we are proud of and will continue to build upon.
Guardian M10 is designed to be intuitive and easy to use, which is crucial in fast-paced clinical environments. Healthcare professionals from both the emergency department and hospital wards have been involved in developing the user interface, resulting in a system tailored to their needs. The staff finds the technology user-friendly and easy to learn. The solution is flexible and can be used in emergency departments, hospital wards, and community settings, improving resource utilization and patient safety. In the long term, Guardian M10 has the potential to replace interval-based systems with continuous monitoring.
The implementation of the solution for the first customers will begin this fall, and projects are currently underway in several countries.
About Vitalthings
Vitalthings was established in 2017 by three experienced technology entrepreneurs/leaders (Ole Johan Ellingsen, Alf-Egil Bogen, and Bård Benum). The company focuses on contactless health technology and works closely with both Norwegian and global clinical and research communities. The organization has 30 employees in Trondheim and Tønsberg and covers the entire value chain, from sensor technology, signal processing, AI, and digital services to clinical and regulatory healthcare expertise. The company has a welfare technology product on the market, a digital night watch, and has recently developed the world’s first contactless patient monitor for continuous monitoring.
Contacts
Bård Benum (CEO)
bb@vitalthings.com
481 64 670
Ole Kristian F. Thu (Chief Medical Officer) Chief Anesthesiologist, Ph.D.
okft@vitalthings.com
905 65 672